The fish, Nothobranchius furzeri, is a short-lived vertebrate of 9 weeks mean longevity. It has been shown that its short life can be modulated using treatment with resveratrol ( » 30% increase in mean longevity). N. furzeri display a decrease in spontaneous locomotor activity and cognitive ability with advancing age that is tied to the development of neurofibrillary tangles. Resveratrol demonstrated an unexplained activity whereby it prevented the development of neurofibrillary tangles and preserved cognitive and spontaneous locomotor activity that control fish lost with age. The author proposes a longitudinal study of neurodevelopment and degeneration of the retina through to the optic tectum in aging N. furzeri under normal conditions and treatment with resveratrol and rooibos tea. Neurodevelopment will be studied in respect to the expression of a suit of cell surface receptors and ligands that have been implicated in axon growth, and how this continued development proceeds into neurodegeneration with age. Degeneration will be investigated through monitoring (1) the accumulation of lipid peroxidation products (lipofuscin) in the brain; (2) activation of astrocytes and microglia; and (3) changes in expression and abundance of a suit of transcription factors and other proteins involved in cellular signaling. Such a study is hampered in other vertebrate models on account of their longer lifespan. It is hoped that this study will reveal insights into aging associated neurodegeneration as well as how resveratrol retards it. Rooibos tea has been shown to have biological activities in common with resveratrol. This research would reveal if rooibos tea has any meaningful neuroprotective benefit in N. furzeri.
Alzheimer's Disease (AD) is a leading cause of death among people 65 years of age and older [Pascale et al., 2007]. AD (as well as other forms of dementias), cancer and heart disease are most prevalent among individuals of the 60 to 80 year old demographic. In western society, on account of a declining birth rate and advances in medical society, this demographic is increasing disproportionately to the younger economically active populations [Chenais, 1990]. In subsaharan Africa the same trend is developing, but instead due to HIV/AIDS [Mathers et al., 2001]. This threatens severe social and economic problems for the future, particularly in countries where a large proportion of the population is dependent on state welfare [Chenais, 1990].
Current aging research, aimed at extending functional human lifespan, is hampered by the limitations of the model organisms available. The short-lived C. elegans and Drosophila do not possess a physiology like unto our own. The rodent and simian models, while having much of our physiology in common, live too long to allow for rapid advancement of this field of research. N. furzeri possess a physiology and genetics more similar to our own than C. elegans and Drosophila and a short enough life span allowing for rapid screening of putative lifespan altering pharmaceuticals and the advancement of theories concerning aging. This makes it an ideal model organism for aging research. N. furzeri and its potential in aging research is reviewed in Genade et al. [2005]. Its short lifespan and slow embryonic development confer on it potential to be useful in studying developmental processes.
With advancing age, N. furzeri displayed an increase in senescence associated b-galactosidase [Genade et al., 2005], a marker of aging that is linked with tumorgenesis [Krtolica & Campisi, 2002]. There was also an increase in lipofuscin in the liver with age [Valenzano et al., 2006]. Lipofuscin, a product of lipid peroxidation, is a well characterized biological marker of aging [Montine et al., 2002]. Of greater significance is the report of a decline in spontaneous locomotion and cognitive ability with advancing age in N. furzeri; and the development of neurofibrillary tangles [Valenzano et al., 2006]. This is mirrored in humans, who with age become less active and develop dementias ranging in severity from simple forgetfulness and an inability to concentrate to debilitating insanity.

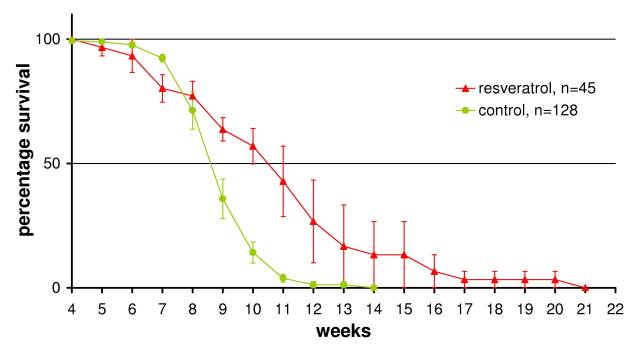
Resveratrol, a naturally occurring polyphenol commonly ingested along with wine (cranberries, peanuts etc...), has been observed to exert many positive biological effects [Granados-Soto, 2003]. It has been shown to extend life-span in Saccharomyces cerevisiae, C. elegans and Drosophila [Wood et al., 2004]. Our research in Italy was the first to demonstrate resveratrol's life-extending effects in vertebrates (Figure 1) [Valenzano et al., 2006]. Dosing at 120 mg/g fish, resveratrol was able to increase life-expectancy from 8.5 to 11 weeks (a 30% increase) and increase maximum life-span from 13 to 20 weeks (a > 50% increase). Along with an increase in life-expectancy there was a rescue of spontaneous locomotion and cognitive ability combined with retardation of neurofibrillary tangle development.
Shortly after our publication Baur et al. [2006] published similar results for mice, confirming the efficacy of N. furzeri as a model organism for vertebrate aging. Since then, Dr. Cellerino has repeated calorie restriction and methionine deficiency experiments originally performed in mice [Sinclair, 2005,Miller et al., 2005] with N. furzeri and has shown it to behave as per mice (pers comm).
The development of the neurofibrillary tangles (NFTs) within N. furzeri is of particular interest because of their association with the development of dementia. These form as a consequence of hyper-phosphorylation of the protein tau [Iqbal & Grundke-Iqbal, 2007,Ferrer et al., 2005]. Properly phosphorylated tau (3 phosphates per molecule) is needed to organize and stabilize microtubules. Hyperphosphorylated tau (6-12 phosphates per molecule) is unable to stabilize the microtubules, giving rise to the formation of paired helical filaments in axons (that form the aggregates referred to as NFTs) and neurophil threads found in dystrophic dendrites [Iqbal & Grundke-Iqbal, 2007]. These taupathies are a hallmark of AD and many other dementias. Even though high concentrations of amyloid-b may be present, dementia only develops in the presence of advanced NFTs [Delacourte et al., 1999]. NFTs have been found in the brains of all people over 75 years of age and in brains as young as 45 years. The hippocampal region of the brain is especially vulnerable.
The development of NFTs are linked to the unregulated activity of kinases within the neural cells [Iqbal & Grundke-Iqbal, 2007] as well as a decline in protein phosphatase 2A (PP2A) concentration and activity [Liu et al., 2005,Wang et al., 2007]. PP2A is the phosphatase chiefly responsible for dephosphorylating tau [Liu et al., 2005] as well as regulating cell signaling through dephosphorylating mitogen-activated protein kinases (MAPK) [Wang et al., 2007]. The decline in PP2A activity is linked to an increase glucose synthase kinase 3 (GSK-3) activity [Wang et al., 2007,Ferrer et al., 2005] as well as an increase in the activities of MAPKs such as extracellular signal-regulated kinase 1 and 2 (ERK 1/2), p38, c-Jun N-terminal kinase (JNK) [Kins et al., 2003] and cAMP regulated protein kinase A (PKA) [Wang et al., 2007] which have all be observed to phosphorylate tau and are linked with the progression of the pathology.
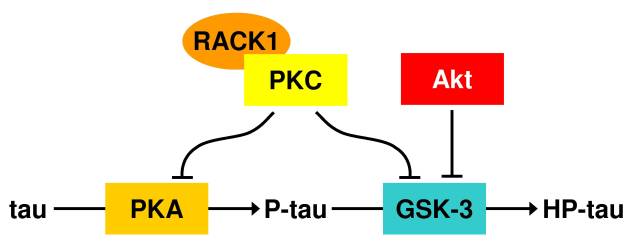
PKA is suspected of priming tau for hyperphosphorylation by GSK-3 (Figure 2) [Iqbal & Grundke-Iqbal, 2007]. GSK-3 is the candidate kinase most likely responsible for taupathies. GSK-3 expression in Drosophila was required to cause the development of advanced taupathies [Jackson et al., 2002]. The activity of GSK-3 is regulated by Akt through phosphorylation at Ser-9 [Ferrer et al., 2005]. Using confocal microscopy, activated Akt has been seen to co-localize with GSK-3 in the vicinity of hyperphosphorylated tau. This links the PI3K-Akt signaling cascade with taupathy development. This same cascade is linked with longevity signaling to the FoxO transcription factors [Lam et al., 2006]. The activation of FoxO transcription factors by resveratrol resensitizes PI3K-Akt [Ni et al., 2007] signaling among many other functions such as up-regulating the expression of several cellular anti-oxidant enzymes such as catalase and Mn superoxide dismutase [Carter & Brunet, 2007].
Like Akt, protein kinase C (PKC) is also a negative regulator of GSK-3 activity [Pascale et al., 2007]. In AD brains there is a decline in PKC activation that is linked to a decline in the scaffolding protein RACK1 that ferries activated PKC to cellular targets [Pascale et al., 2007]. RACK1 also ferries cAMP-specific phosphodiesterase-4D5 linking it to the regulation of cAMP levels, and thus the suppression of PKA activity [Lynch et al., 2007]. In addition to PKA and calmodulin, PKCa has been shown to activate phosphodiesterase [Cai & Lee, 1996]. Another PKC chaperone, HSP70, has also been observed to decline with age [Bastianetto et al., 2007]. It serves to stabilize PKC. Without HSP70, PKC will interact randomly with the cytoskeleton [Ameyar et al., 2003]. The activation of sirtuins results in the activation of the cell-survival properties of the human FoxO3a (and C. elegans ortholog DAF-16) transcription factor [Lam et al., 2006,Wood et al., 2004]. This causes an up-regulation in HSP70 expression [Carter & Brunet, 2007]. Resveratrol activates sirtuins [Wood et al., 2004] and has been shown to restore PKC activity [Salminen et al., 2008]. Interestingly, Wolkow et al. [2000] showed that DAF-16 activity is only needed in the neuronal cell lineage of C. elegans in order to confer increased longevity.
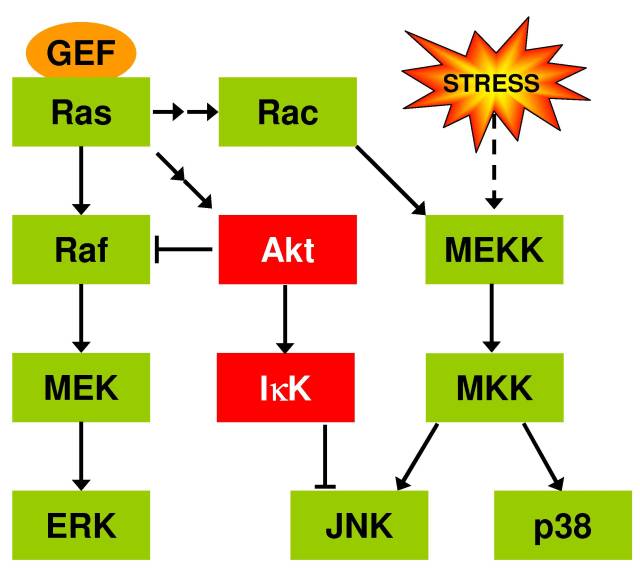
Resveratrol, through the activation of sirtuins, can suppress the activation of NF-kB [Stefani et al., 2007] which is implicated in the inflam-aging hypothesis of aging where the dysregulation of the innate immune system (giving rise to unchecked inflammation) causes the pathologies associated with aging [Salminen et al., 2008]. In aging rats it has been observed with advancing age there is an increase in activated astrocytes and microglia [Kim et al., 2004]. The activated microglia are a source of reactive oxygen species (ROS) [Sansoni et al., 2008,Salminen et al., 2008] while the activated astrocytes over-produce a ligand, S100B, that has been shown to activate GSK-3 and JNK in neuronal precursor cells [Esposito et al., 2008]. JNK (and p38) are also activated by MEKK in response to cellular stress (such as caused by ROS) [Kins et al., 2003]. JNK can activate AP-1 (through phosphorylation of c-Jun) [Ameyar et al., 2003] as well as activate FoxO3a [Lam et al., 2006] while inhibiting survival signaling though the PI3K-Akt pathway [Salminen et al., 2008]. The combination of FoxO3a and AP-1 activation by JNK puts into operation cell death programs that the additional activation of FoxO3a by sirtuin can turn into cell survival/stress resistance/repair programs [Lam et al., 2006,Brunet et al., 2004]. It is interesting to note that while resveratrol has not been shown to alter Ras-MAPK signaling, it does result in the down-regulation of the small guanine nucleotide exchange factor, Ras-GFR1, which is AP-1 inducible [Stefani et al., 2007] suggesting that resveratrol promotes the survival/repair programs by suppressing AP-1. Recalling that FoxO3a activity can resensitize Akt, Akt can suppress JNK activation through its activation of IkB kinase [Salminen et al., 2008].
|
|
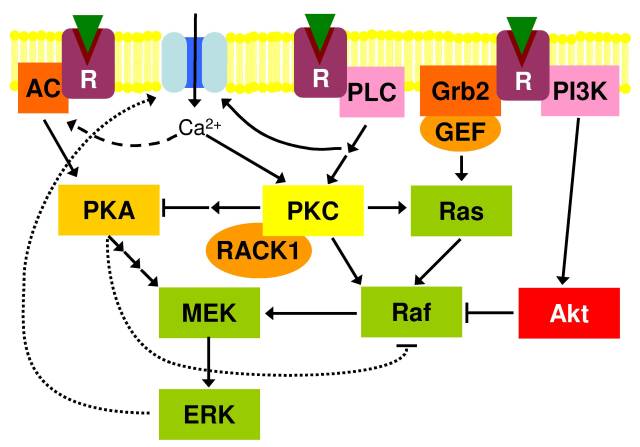
Figure 3: Interaction between PKA, PKC and Ras signaling. Grb2 is a scaffolding protein linking receptors (R) to guanine nucleotide exchange factors (GEF). AC, adenylyl cyclase; IkK, IkB kinase; PLC, phospholipase C; PI3K, phosphoinositide 3-kinase.
Figure 3 summarizes the afore mentioned interactions as well as introduces those of PKA and PKC with Ras-MAPK signaling. PKA and PKC can both activate CREB and interact with Ras-MAPK signaling (which cross-talk with MEKK signaling) [Roberson et al., 1999]. PKA and PKC have important roles in the response of the axon growth cone to extracellular cues [Goldberg, 2003] and are vitally important in memory formation and neural plasticity [Pascale et al., 2007]. PKC activity has been shown to be neuroprotective [Pascale et al., 2007]. Both PKC and PKA activation can lead to the activation of ERK and its localization to the plasma membrane though not by the same route [Roberson et al., 1999]. PKA inhibits the Ras-Raf pathway but activates MEK directly; while PKC directly activates Ras and Raf. The PKA pathway thus functions free of the inhibition by Akt -that inhibits Raf-1 through phosphorylation [Kolch, 2000]-while the PKC pathway would still be susceptible to it. Both PKA and PKC activity has been shown to activate ERK 1/2 in the hippocampus [Roberson et al., 1999].
Activated ERK can reinforce the activation of both PKA and PKC by phosphorylating ion channels and receptors in the plasma membrane [Fioretti et al., 2006]. The influx of Ca2+ can directly activate PKC and CaM Kinases which in turn can activate adenylyl cyclase to activate PKA [Roberson et al., 1999]. With the combination of an age related decline in PP2A activity and RACK1 expression any signal dysregulation could rapidly spiral out of control. Both PKA and ERK can phosphorylate tau.
Axon guidance is facilitated though repulsion and attraction signals received by the growth cone and generated by the surrounding tissue [Goldberg, 2003]. These signals activate PKA, PKC, Ras-MAPK as well as Wnt signaling which employs GSK-3 [Ferrer et al., 2005,E.R. et al., 2000,Goldberg, 2003]. Brain areas such as the hippocampus of humans [Sairanen et al., 2005], and the optic tectum of fish are dynamic with new neurons and glia being generated and new connections being forged [Lang et al., 2001]. It may be no coincidence that it is in these dynamic brain areas that taupathies develop, and so explaining why even though in the case of AD amyloid-b deposits spread randomly [Delacourte et al., 1999]. The expression of axon guidance receptors has been shown to change with age/developmetnal stage [Goldberg, 2003,Lang et al., 2001,Dali et al., 2006]. Further more, the extracellular matrix (ECM) has been shown to deteriorate with age [Jenkins, 2002] and many of the axon guidance cues are imbedded in the ECM. It could be that neuronal cells that develop and extend axons later in life may not be able to communicate with older cells resulting in signal cascade malfunction, innate immune system activation and taupathies.
Lipofuscin is a well characterized lipid-peroxidation product and biomarker of aging [Montine et al., 2002]. It is probable that lipofuscin accumulation can lead to a change in membrane fluidity and permeability [Ran et al., 2007]. This can affect the activity and cooperation of the cell surface receptors (such as Eph, p75NTR and Trk) that activate kinases within the axon [Teng & Hempstead, 2004]. Changes in membrane permeability can affect depolarization of the cell and thus activation of CREB (via PKA and PKC) that is ordinarily needed to sustain and guide the growing axons [Goldberg, 2003]. It is interesting to note that young rats can clear lipofuscin from their membranes while old rats cannot [Inanami et al., 1995].
N. furzeri allows us to explore the development of taupathies in all the above discussed contexts. Further more, the discovery that resveratrol retards NFT development in N. furzeri allows us to examine the significance of the roles of inflam-aging, intercellular communication failure and membrane deterioration in NFT development. In addition, the observation that rooibos tea prevents lipofuscin accumulation [Inanami et al., 1995] could enable us determine the role of lipofuscin in aging and neurodegeneration where lipid peroxidation has been associated with AD [Montine et al., 2002]. Whether rooibos tea can increase the longevity of N. furzeri is also interesting. The answer to this question would emerge as a biproduct of the research into NFT development.
The aim of this research is to learn more about the development of NFT in N. furzeri and how resveratrol (and perhaps rooibos tea) affects it. Four interrelated topics will be explored:
Lipofuscin is a well characterized biomarker of aging as previously discussed. N. furzeri accumulates lipofuscin with age but resveratrol was not shown to affect this [Valenzano et al., 2006]. However, lipofuscin content was only monitored in the liver not the brain.
It is important to determine whether lipofuscin accumulates in the aging brain and whether resveratrol prevents this. Also of importance is whether only old cells accumulate lipofuscin. The observation that young rats can clear lipofuscin from their membranes while old rats cannot inspires the question as to whether lipofuscin accumulation is a gradual intrinsic process (the product of natural wear and tear) or a symptom of another pathology such as innate immune system hyper-activity (i.e. inflam-aging) that generates more lipofuscin than can be removed.
It is possible to distinguish between recently developed retinal ganglion cells (RGCs) and their axons from established RGCs by labeling them with Neurotrace. This will also enable us to determine the generation time of RGCs. Knowing the generation time of the RGCs and other cells in the retina-optic tectum system is of interest in itself. It has been observed in rats that with advancing age there is a decline in the capacity to regenerate RGCs [Goldberg, 2003]. It would be interesting to determine the generation time and correlate it with the accumulation of lipofuscin and glial cell activation.
It stands to reason that the ROS generated as consequence of inflam-aging will cause an increase in lipid peroxidation and thus lipofuscin accumulation. Lipofuscin accumulation should therefore only accelerate after the dysregulation of the immune system. This immune system dysregulation is typified in the CNS by an increase in activated astrocytes and microglia [Kim et al., 2004]. Kim et al. [2004] has shown that there is a decline in activated astrocytes and microglia in calorie restricted rats compared to controls. They also showed that following ischemic insult there was also less inflammation and cell death in the calorie restricted rats. Resveratrol has been shown to mimic the effects of calorie restriction [Wood et al., 2004].
It would be interesting to see if resveratrol can reduce astrocyte and microglial activation and whether this will also retard the amount of lipid peroxidation and development of NFT. Rooibos has been shown to prevent lipid peroxidation in rats. Is this due to it preventing the onset of inflam-aging? This can be determined by comparing lipofuscin accumulation and glial activation in young and aged brains of normal, resveratrol and rooibos treated N. furzeri. Rooibos tea has been shown to modulate the immune system and alter the levels of glutathione [McKay & Blumberg, 2007]. Glutathione is implicating in reducing oxidized membrane lipids [Ran et al., 2007]. Activated astrocyte secrete S100B that is implicated in causing NFTs. Determining whether N. furzeri astrocytes secrete S100B and how this changes with age and treatment with resveratrol would also be informative.
It is probable that lipofuscin accumulation can lead to a change in membrane fluidity and permeability [Ran et al., 2007]. This can effect the activity and cooperation of the cell surface receptor kinases and their detection of the ligands in the ECM [Teng & Hempstead, 2004]. In this way the normal development of the nervous system can turn pathological with advancing age.
The author intends to investigate the aging related expression patterns of L1 (E587) and TAG-1 over time in N. furzeri. These neural markers of axon growth and development (and those mentioned below) have been shown to change in respect to expression location and abundance with age [Stuemer & Bastmeyer, 2000,Bernhardt, 1999,Lang et al., 2001]. The oligodendrocytes cells will also be monitored through staining for O4 and tenascin-R [Lang et al., 2001]. The latter, produced by oligodendrocytes, is implicated in modulating the activities of mammalian microglia (particularly by repelling them from axon bundles) and in stimulating precursor cells to differentiate into oligodendrocytes [Liao et al., 2005]. The expression of integrins on growth cones and their associated binding partners: fibronectin and laminin-2 in the extracellular matrix will also be investigated. The cellular response to the interaction of the above markers involves Wnt signaling pathways which utilizes GSK-3 [Pollard & Earnshaw, 2002].
The localization of these receptors and ligands change through developmental stages and time [Goldberg, 2003,Lang et al., 2001,Dali et al., 2006]. It is possible that neurons that develop later in life, after the established neurons and glia have down-regulated their surface receptors and signaling complexes, do not receive the correct signals from established neurons and glia resulting in errant growth that could culminate in aberrant kinase activation resulting in tau hyperphosphorylation and the development of NFTs in the axons.
Furthermore, as many of the functions of ligands and receptors are still of uncertain function, this line of research could produce valuable insights into neurodevelopment.
There are seven proteins of interest: four transcription factors and three cell-signaling support proteins. These are the transcription factors (subunits) c-Jun (of AP-1), CREB, FoxO5 (the fish ortholog of FoxO3a [Rudd et al., 2003]) and c-Rel (of NF-kB). The roles of these factors have been discussed in section 1.2; and the proteins PP2A (which has been observed to decline with advancing age and NFT pathology), RACK1 and HSP70 (proteins critical for correct PKC activity).
The localization of these and their relative quantities will be compared though the aging process and between control and resveratrol and rooibos tea treated fish. No change in the abundance of PP2A between control and experimental fish would suggest that PP2A decline could be a red-herring in NFT research.
As resveratrol has been shown to affect PKC activity and HSP70 expression the monitoring of the combination of RACK1 and HSP70 expression along with CREB could reveal whether PKC is involved in N. furzeri neuroprotection. In zebrafish a small change in the activity of CREB can lead to developmental abnormalities of the nervous system [Dworkin et al., 2007]. PKA also activates CREB, so any aberrations in CREB activity with age and between control and experimental fish that are independent of changes of RACK1 and HSP70 could support the hypothesis that PKA is involved in NFT development and PKC is not.
NF-kB is activated by PI3K-Akt signaling while FoxO is deactivated by it. NF-kB activity is linked with inflam-aging while FoxO activity is linked with life-span extension. The activation of FoxO along with AP-1 sets into motion the cell's death programs, where as FoxO activation by sirtuins sets into motion the cell's survival and repair programs. With advancing age one should expect to see a gradual increase in NF-kB activation (translocation to the nucleus) in accordance with the inflam-aging theory. This will result in an increase in ROS that will activate JNK. JNK will then activate AP-1 and FoxO, thus activating the cell's death programs. Resveratrol should result in both a decrease in NF-kB signaling as well as AP-1 while activating sirtuins and the cell's FoxO3a mediated survival/repair programs.
If resveratrol and rooibos do reduce NF-kB signaling, a decline in glial activation should be observed. If this corresponds with a decline in NFTs, this could be regarded as supporting evidence for inflam-aging causing NFTs. The persistance of NFTs in the absence of inflam-aging would suggest that the pathology (in N. furzeri) has its root elsewhere-for instance in uncoordinated cell-cell signaling during development.
It is clear that this is an ambitious project and it may not be
feasible to address all these aims in the space of three years.
Never the less, the advancement of our understanding of only one
of these topics of interest would already be note-worthy. Even if
each topic is only touched on, it would generate many avenues for
further research. The choice of which topic to concentrate on will
depend on the suitability of fish to the following methods.
The retina, a well characterized portion of the CNS, will be used for this study. The development and degeneration of the retina through the optic nerve to the optic tectum will be examined in a longitudinal study of aging. Immunohistochemistry and confocal microscopy will be the back bone of this project.
Control fish will be grown in the lab as described by Genade [2005] and fish sacrificed at weekly intervals for the longitudinal study of aging in N. furzeri. Tissue used to determine lipofuscin accumulation will need to be snap frozen to prevent any additional oxidation post mortem. Lipofuscin is detected by excitation at 488 nm. Neurotrace will be used to distinguish between old established neurons and young developing neurons by injection into the eyes of 7 week old fish (i.e. one week before the 50% population survival point, see Figure 1). The quantity of lipofuscin in established and young Neurotrace labeled neurons can then be compared. The Neurotrace can also be used to determine whether the NFTs observed in the optic tectum are caused by young neurons extending into the tectum as well as the generation time of RGCs.
To determine the generation time of neural cells in the fish CNS, BrdU will be added to the water the fish are kept in. Fish will be sacrificed at defined intervals and the number of BrdU labeled cells in the CNS will be correlated with the exposure time in order to calculate the generation time of N. furzeri neural cells. This will be repeated at several ages to determine whether the generation time changes with age.
For the monitoring of glial activation brain tissue will be fixed in paraformaldehyde and stained with lectin and iso-lectin to expose activated microglia. Activated astrocytes will be exposed though the use of GFAP antibodies-which has already been shown to work in N. furzeri (Cellerino, pers comm). Fixed sections will also be used for staining with antibodies to evolutionary conserved epitopes of CREB, c-Rel, FoxO3a, HSP70, PP2A, phospho-c-Jun and RACK1. NFTs will be monitored using Flouro-Jade.
Frozen and unfixed tissue will be used for the determination of the location and abundance of ECM ligands and cell-surface receptors on account of the cross-linking action of paraformaldehyde.
Tissue sections and whole-mounts from normally aging N. furzeri will be compared to sections taken from N. furzeri treated with resveratrol and rooibos tea. Dosing with resveratrol has been previously described [Genade, 2005] but a dosing protocol will need to be developed for rooibos tea. Initially, rooibos tea bags will simply be added to the tank water as the topical application of rooibos tea has previously been shown effective [Marnewick et al., 2005]. If rooibos has no effect on life-span its use will be restricted to investigating the role of lipofuscin in neurodevelopment/degeneration (assuming it has any affect on N. furzeri lipofuscin accumulation). Initially, only sections taken from young (4 week) old and aged fish (where 50% of the population survives) of the resveratrol and rooibos treated fish will be be compared to the controls. Differences observed between these young and aged fish and the controls will be examined though a longitudinal study as for the control fish. This is to save time as well as expensive reagents.
As these fish can be spawned in large numbers and the their lifespan is short, many experiments can be performed in one year and samples gathered. The work discussed in section 2.1 and 2.2, comparing normally aging fish and resveratrol treated fish, could be completed within 12 months as this work rests on well established techniques using reagents that are known to work in fish. The work in sections 2.3 and 2.4 could be more problematic as the available antibodies may not recognize the fish epitopes. A ``shotgun'' approach will be used to probe the fish and see what ``sticks''. This study would take much longer and will run concurrently with the work in sections 2.1 and 2.2 which are the principal focus of the research.
Resveratrol has been shown to extend the life-expectancy and life-span of N. furzeri. This extension in life-expectancy is linked to a retention of spontaneous locomotion, cognitive ability and retardation of NFT development. Rooibos tea has been shown to possess many of the biological properties of resveratrol [McKay & Blumberg, 2007]. The development of taupathies, are a hallmark of many aging-related dementias. It is hoped that by studying the development of taupathies in N. furzeri, and how resveratrol (and perhaps rooibos tea) is able to retard them, insight may be gained into their development (around which there is still uncertainty). It is further hoped that these insights could be exported to other model organisms (and perhaps humans), and that they may lead to the development of cheap and efficient therapies to counter the pathologies associated with aging, giving us a longer and fitter life.
References